An over-active thyroid due to Graves’ disease occurs in up to 1 in 100 women in the reproductive age range. Up to half of patients with Graves hyperthyroidism can be affected by Thyroid eye disease (TED). However some patients with TED can have a normal or underactive thyroid gland.
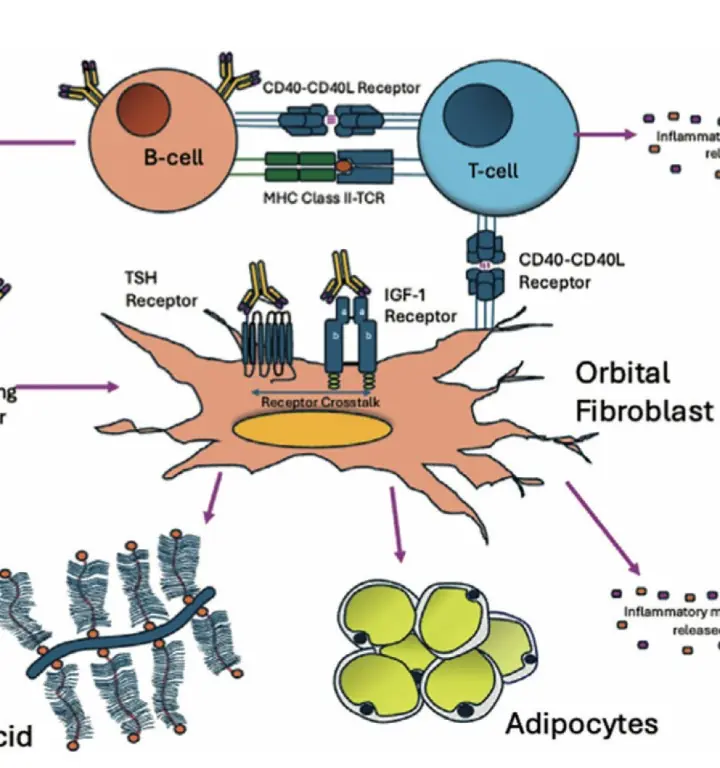
TED is commonly associated with bulging forward eyes (exophthalmos) and eyelid retraction ( raised upper eyelid). . TED can cause physical discomfort including eye pain, grittiness, excessive watering and light sensitivity. TED also causes significant inflammation inside the eye socket (orbit) leading to swelling of the muscles that move the eyes and increase in fat deposits. As the orbit is a confined space this can cause the eye to be displaced forwards as well as caused double vision as the eyes don’t move together in sync leading to double vision. These changes often cause profound psychological distress that can severely blight patient’s lives.
However, many TED patients with significant disfigurement are classified as having ‘mild’ clinical disease. This is partly because there has been no effective treatment available for this group of patients apart from stabilising their overactive thyroid levels, using soothing eyedrops and taking Selenium supplements.
Approximately 1 in 4 TED patients develop disabling double vision that can stop them from driving and/or severe inflammation causing constant pain and swelling that would benefit from immunosuppression treatment to treat the underlying inflammation. Up to 1 in 20 can suffer significant visual loss that require high dose immunosuppression and sometimes urgent surgery.
Successful TED treatment should be able to quickly reverse visual problems and disfigurement, have a good safety profile and improve patient’s quality of life. Unfortunately, no treatment that fulfils all the above criteria is currently available outside the USA.
In Europe and most of the world patients with moderate to severe TED are offered either intravenous or oral steroids and other treatments to suppress the immune system. These treatments are reasonably safe if monitored closely, and effective in preventing vision loss and relieving pain and swelling, and sometimes improve double vision. However, these treatments do not usually reverse the exophthalmos (shrink the eyeballs backwards into the eye sockets).
Significant exophthalmos is usually treated by orbital decompression surgery (breaking open the eye socket walls to enlarge the orbital cavity to allow the eye to settle backwards). Most doctors do not recommend this major surgery to improve the exophthalmos until the orbital inflammation has settled and the hyperthyroidism has been effectively and permanently treated. This is to reduce the risk of the TED flaring up again after the surgery. As a result, many TED patients face years of living with their disfigurement before having this surgery.
However there has been exciting new treatment developments. Tepezza ™ has been approved for use in TED in the USA since 2019 and has been very successful in reversing disfigurement and inflammation quickly in many TED patients who did not go on to need orbital decompression surgery.
While Tepezza is not available outside the USA, many drug companies are now developing new treatments for TED. Some of these new drugs work in a very similar way as Tepezza and some others by novel mechanisms thanks to new scientific understanding of the causes of TED. Patients can now get access to these new treatments by taking part in clinical trials now being conducted in the UK.
If you want to participate in these trials, you should discuss with your eye specialist for a referral to these trial centres. Details can be found on https://clinicaltrials.gov
What is a Clinical Trial?
A clinical trial is a research study designed to evaluate new treatments, medications, or procedures. These trials aim to determine the safety, effectiveness, and potential side effects of new therapies before they become widely available.
Why Participate in a TED Clinical Trial?
- Access to cutting-edge treatments before they are widely available.
- Contribute to medical research that may benefit future TED patients.
- Receive specialized care from a team of experts.
- Regular monitoring and comprehensive health assessments.
Understanding the Phases of a Clinical Trial
- Phase 1: Small group testing to evaluate safety and dosage.
- Phase 2: Larger group testing to assess effectiveness and monitor side effects.
- Phase 3: Comparison of the new treatment with standard care in a larger population.
- Phase 4: Post-approval monitoring of long-term effects.
What to Expect as a Participant
1. Informed Consent Process
Before enrolling, you will receive a detailed explanation of the trial, including potential risks and benefits. You have the right to ask questions and withdraw at any time.
2. Eligibility Assessment
You may need to undergo medical tests and screenings to determine whether you qualify for the trial.
3. Treatment and Monitoring
- You may receive the investigational treatment or a placebo (in some trials).
- Regular check-ups, blood tests, imaging, and other assessments will be conducted.
- You may need to keep a journal of your symptoms and experiences.
4. Potential Risks and Side Effects
- Novel treatments may cause unexpected side effects.
- Some treatments may not be as effective as existing options.
- Participation may require more frequent hospital visits.
Clinical Trial Availability and Recruitment
- The timing of recruitment for clinical trials is variable, meaning availability to participate cannot always be controlled.
- For some trials, if you give consent to allow your contact information to be passed onto the trial coordinator, our team will contact you when a potentially suitable trial becomes available.
- You will receive then a Patient Information Sheet (PIS) from the trial coordinator via email. If you are interested, you should reply to indicate that you would like more information—this does not commit you to participating.
- If you do not respond to the email , we will not contact you further about this particular trial.
Your Rights as a Participant
- Voluntary Participation: You can withdraw at any time without affecting your standard care.
- Confidentiality: Your medical information will be protected.
- Access to Information: You will be informed about trial progress and any findings relevant to your health. However your investigator and yourself will not usually have information about which study arm you are assigned to unless it is an ‘Open Label Extension’ trial.
- Compensation: Most TED trials reimburse expenses, but participants are not paid for their participation.
How to Prepare for Participation
- Discuss the trial with your doctor (GP/ endocrinologist) and loved ones.
- Prepare a list of questions, such as:
- What are the potential benefits and risks?
- How will the trial impact my daily life?
- Will I need to stop my current medications?
- What happens if I develop side effects?
- Keep an open line of communication with the research team.
Your participation could make a difference in the future of TED treatment!